Project Summary
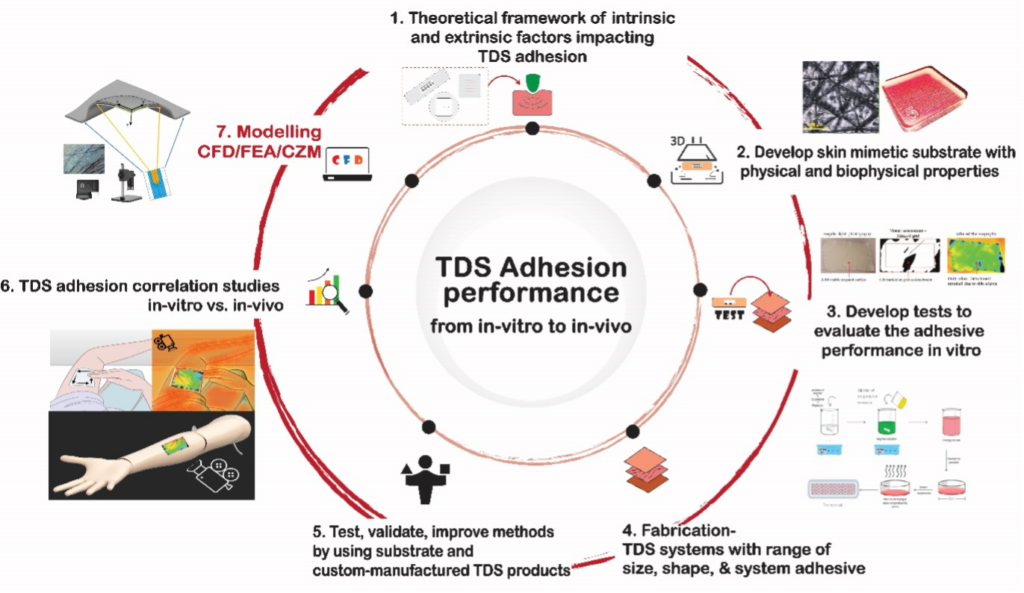
In vitro adhesion performance of transdermal delivery systems (TDS) and its correlation to in vivo adhesion is currently challenging. This project aims to cover a wide range of key developmental issues, failure modes, and mitigation strategies to overcome adhesion failures in TDS. The first step, will develop a theoretical framework of all the intrinsic and extrinsic factors to the TDS, that influence their adhesion performance in vivo. The next steps will involve identifying and manufacturing suitable skin mimetic substrates for in vitro adhesive performance test. For this, a set of custom-manufactured TDS (cTDS) formulations will be developed that systematically vary their intrinsic properties. To assess adhesion, the project will develop novel adhesion test methods with improved accuracy, reproducibility, and in vivo correlation to establish “adhesive equivalence” between reference and generic TDS. Finally, in vitro and in vivo adhesion studies will be conducted with varied TDS, under controlled and harmonized conditions, using newly developed skin mimetic substrate(s) to assess in vitro – in vivo correlation (IVIVC) as well as, the capacity of the newly developed in vitro tests, to be predictive of in vivo adhesion. The outcomes of this project are expected to provide accurate tools, in the form of a fully integrated and validated in vitro test for adhesion, that is capable of replicating a myriad of real in use scenarios a TDS undergoes when applied by a patient.