Project Summary
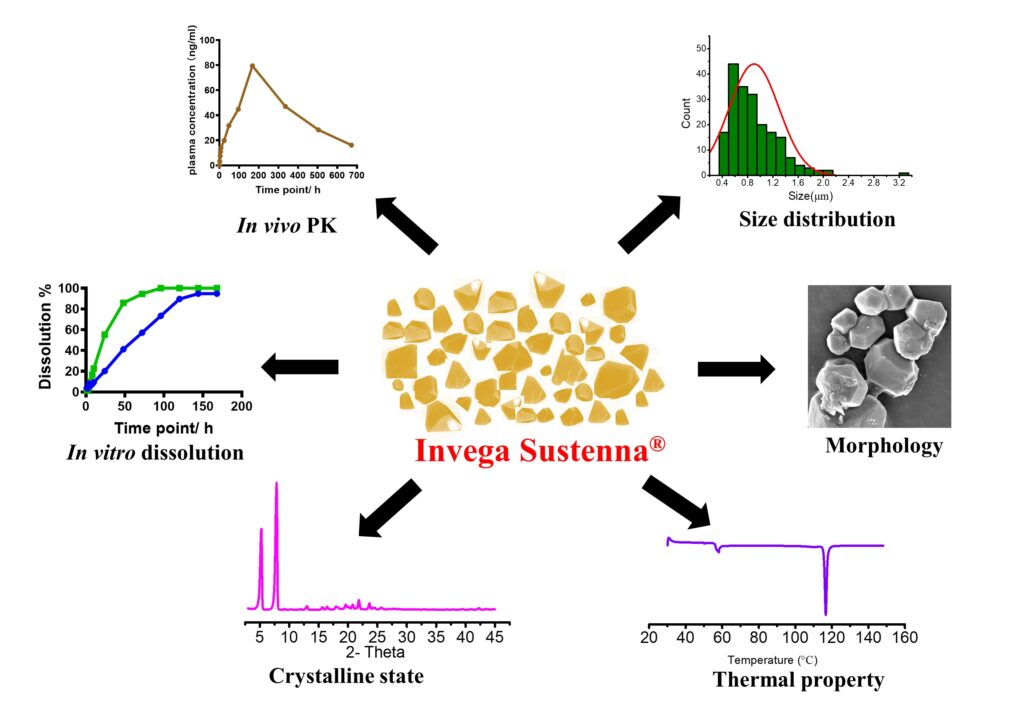
Paliperidone palmitate (PP) is a second-generation dopamine-2 receptor antagonist used for the treatment of schizophrenia. Currently, there are three LAI suspensions of PP approved by Food and Drug Administration (FDA), developed and commercialized by Janssen Pharmaceutical Company, Invega Sustenna®, Invega Trinza® and Invega Hafyera®. These formulations have administration intervals of every month, every three months, and every six months, respectively. The decrease of dose frequency results from the increased particle sizes and drug concentration.The aqueous suspension formulations generally have less excipients, and could avoid gastrointestinal absorption and first-pass effect through intramuscular injection. Moreover, due to the low solubility of PP, the aqueous suspension can achieve long-term therapeutic effects at a controlled drug release rate, allowing for low dose frequency, low recurrence rate and higher patient adherence. However, there is no generic products of PP long-acting injectable suspension on the market. The development of generic product of Invega Sustenna®, not only enriches treatment options for patients but also would contribute to reduce healthcare burden. In generic drug development, demonstration of bioequivalence is required to evaluate therapeutic equivalence. The in vitro and in vivo performance, which demonstrates bioequivalence, is decided by critical quality attributes of the formulations, such as size distribution, morphology, thermal characteristics and crystalline properties. Therefore, the effectiveness and safety of the final generic products heavily rely on the physical and chemical properties of the formulations.
In this project, we will establish a systematic quality characteristics assessment for Invega Sustenna®, including particle size distribution, particle morphology, thermal characteristics, crystalline properties, in vitro dissolution kinetics and in vivo pharmacokinetics. Multiple lots of commercial products will be obtained and the quality of long-acting PP suspension (Invega Sustenna®) will be comprehensively characterized through reverse engineering. We have developed a series of analytic methods to assess critical quality attributes and in vitro dissolution methods to evaluate the release behaviors of Invega Sustenna®. The valuable insights obtained from our research will provide critical knowledge and information that will not only aid development and regulatory assessment of generic long-acting PP injectable suspensions but also holds significance for differentiated LAI suspension product development through the 505(b)(2) application pathway.