Project Summary
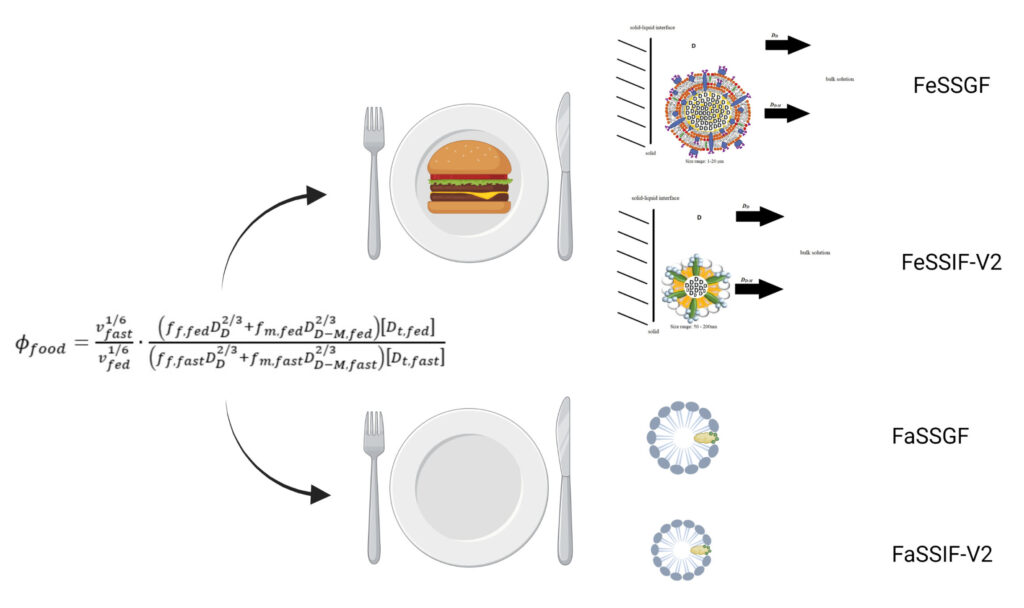
Biorelevant media that employs phospholipid-bile salt mixed micelles have been devised to better mimic the in vivo gut fluid compositions. For example, fed state simulated intestinal fluid version 2 (FeSSIF-V2) mimics fed intestinal composition. Also, fed state simulated gastric fluid (FeSSGF) mimics fed gastric composition. While the existence of phospholipid-bile salt mixed micelles and fat globules to sometimes enhance in vivo drug dissolution is well accepted, a predictive and quantitative understanding is not well developed. The objective was to quantitatively assess the contributions of biorelevant media-mediated solubility and diffusivity on enhanced drug dissolution in FeSSGF and FeSSIF-V2, versus their corresponding fasted state media. This objective was designed to better parameterized physiologically based pharmacokinetic (PBPK) models for oral drug absorption by providing a surfactant-mediated dissolution model of drug that can be incorporated into physiologically based pharmacokinetic (PBPK) models for oral drug absorption.
In studies of fed-state biorelevant media (1), results showed drug-saturated FeSSGF globules and FeSSIF-V2 mixed micelles were large and slow diffusing (diffusivities of about 1×10-9 and 7×10-8 cm2/s, respectively), compared to free drug (about 7×10-6 cm2/s) and drug-bound micelles from pharmaceutical surfactants (about 0.5-1×10-6 cm2/s). Of three drugs, griseofulvin exhibited the greatest biorelevant media-enhanced solubility and dissolution (652-fold and 6.23-fold respectively in FeSSGF, and 190-fold and 12.7-fold respectively in FeSSIF-V2), but slow colloid diffusivity markedly attenuated large solubility benefits, particularly in FeSSGF.
In studies of fasted-state biorelevant media (2), results showed that FaSSGF and FaSSIF-V2 mixed micelles were large and slowly diffusing (diffusivities about 2×10-8 cm2/s and 12×10-8 cm2/s, respectively), compared to free drug (about 7×10-6 cm2/s), although were smaller and more rapidly diffusing compared to drug-bound fat globules and mixed micelles from FeSSGF and FeSSIF-V2 (about 1×10-9 cm2/s and 7×10-8 cm2/s, respectively). Dissolution enhancement in FaSSGF and FaSSIF-V2 was the same as solubility enhancement, which was minimal. FaSSGF and FaSSIF-V2 practically did not enhance drug dissolution, since solubility was at best minimally increased, but also since micelles were relatively slowly diffusing relative to free drug diffusivity.
Fed results were compared to fasted results (3). Relative to solubilization, in vitro dissolution was many fold lower in fed media over fasted media, indicating the compromising role of micellar and fat-globule diffusivity in attenuating dissolution rate based on solubility enhancement alone. Model prediction of food effect were in agreement with observed effects for griseofulvin, ketoconazole, and ibuprofen. The understanding of attenuation of in vitro dissolution in fed versus fasted biorelevant media may contribute towards predicting in vivo food effects, including lack of in vivo food effect for some poorly water soluble drugs. Results and model parameterization has promise for improved PBPK models for oral drug absorption of poorly water soluble drugs.
References
- Jamil R, Polli JE (2022): Prediction of In Vitro Drug Dissolution into Fed-state Biorelevant Media: Contributions of Solubility Enhancement and Relatively Low Colloid Diffusivity. DOI: 10.1016/j.ejps.2022.106179. Eur. J Pharm. Sci. 173:106179.
- Jamil R, Polli JE (2022): Prediction of In Vitro Drug Dissolution into Fasted-state Biorelevant Media: Contributions of Solubility Enhancement and Relatively Low Colloid Diffusivity. DOI: 10.1016 j.ejps.2022.106210. Eur. J Pharm. Sci. 174: 106210.
- Jamil R, Polli JE (2022): Prediction of food effect on in vitro drug dissolution into biorelevant media: Contributions of solubility enhancement and relatively low colloid diffusivity. DOI: 10.1016/j.ejps.2022.106274. Eur. J Pharm. Sci. 177: 106274.