Project Summary
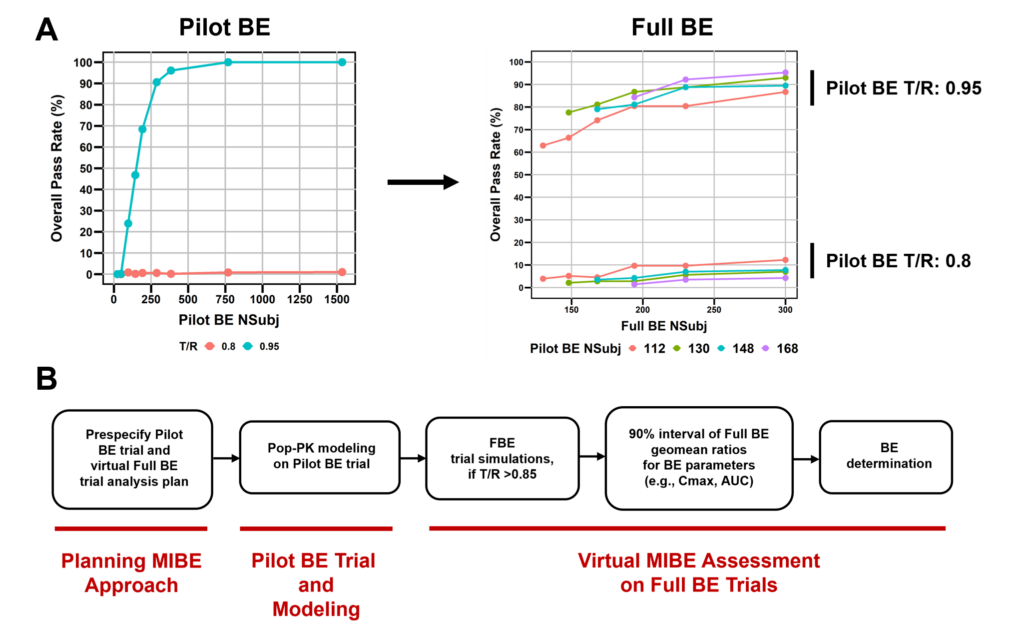
Overall, 90% of prescriptions filled in the U.S. are for generic drugs. However, long-acting injectables (LAIs), which provide many benefits to patients, such as improved adherence due to reduced dosing frequency and decreased incidence of side effects, have generics approved for less than 20% of brand products. Generic drug development for complex drug products can be challenging due to complications related to complex absorption profiles and long apparent half-lives. Innovative trial designs and bioequivalence (BE) approaches that integrate modeling and simulation may be necessary to encourage generic drug development for these products. We propose here a model-integrated bioequivalence (MIBE) approach in which a smaller Pilot BE trial is conducted, while BE is assessed virtually on larger simulated Full BE trials. The reference list drug Depo-SubQ Provera 104 was used to demonstrate the MIBE approach. Single-dose PBE trial simulations were performed for bioinequivalent (T/R: 0.8) and bioequivalent (T/R: 0.95) scenarios. Estimation was performed on Pilot BE trials, and Full BE trials were then simulated. BE was evaluated by determining the geometric mean ratio (GMR) for each FBE simulation repetition and then assessing average BE based on whether the 5th and 95th percentiles of the distribution of GMRs were between the 80-125% BE margins. With this model-integrated approach, we were able to substantially increase BE power while reducing the number of subjects, without compromising Type I error. This MIBE approach provides a framework by which modeling and simulation can be utilized by generic drug companies to accelerate the assessment of BE in products for which BE is difficult to establish by traditional approaches.